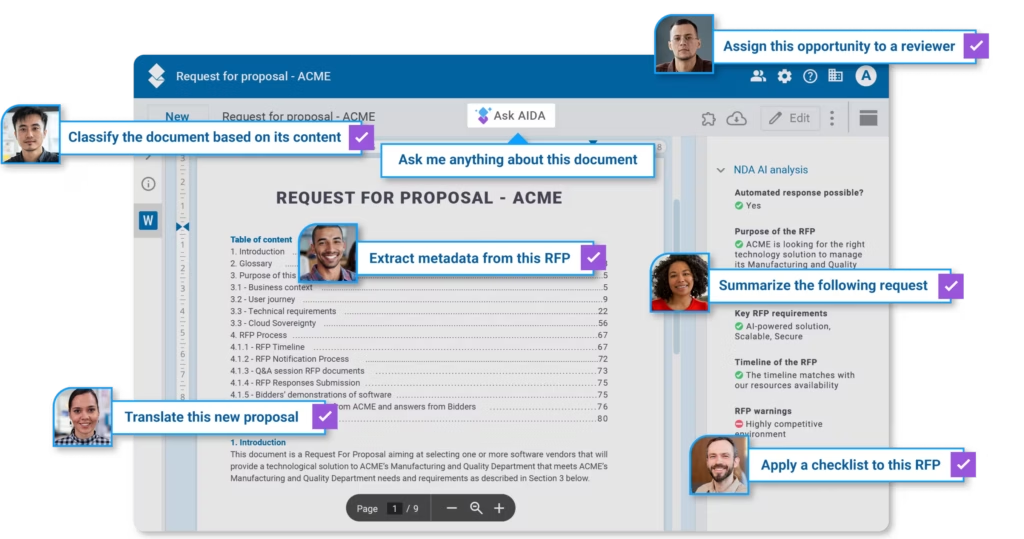
Manual tasks have long slowed down quality teams — from reviewing redundant procedures to verifying employee comprehension for compliance audits. Today, when AI tools are integrated with a new-generation Document Management System (DMS), they may offer a trustworthy and smarter way forward for QMS leaders.
Here are three practical ways artificial intelligence can simplify and accelerate quality management processes managed with a DMS, helping teams focus on improvement rather than administration.
1. Detect and Consolidate Redundant Policies
When policies and procedures multiply over time, inconsistencies and duplication creep in. AI can analyze an entire policy library, highlight overlaps, and recommend how to consolidate multiple documents into one — with full transparency on why each suggestion is made.
By reducing clutter and aligning content, quality teams gain clearer oversight and a leaner, more effective QMS.
2. Review Procedures for Clarity and Improvements
Even well-written procedures can benefit from a fresh pair of eyes. In this use case, AI reviews a document’s language, structure, and clarity, offering specific edits to make content easier to read and apply.
This is especially useful for internal training, onboarding, or audits where procedural clarity is essential.
3. Create Quizzes to Verify Understanding and Support Compliance
Quality standards like ISO 9001 often require proof that employees understand key policies. AI now makes it easy to generate quizzes — such as multiple-choice or true/false questions — directly from a policy document.
This feature allows teams to track comprehension, reinforce learning, and maintain the documentation needed for compliance audits.
Smarter Quality Management Starts Here
These use cases illustrate how AI can support quality professionals by streamlining repetitive work, improving documentation, and enhancing compliance workflows. But how can you translate such principles into actual productivity-boosting measures you can easily put in place? The practical examples are powered by AIDA, the AODocs AI assistant that integrates with companies’ Quality Management Systems. Designed for regulated environments, AIDA offers reliable automation with full control over decisions and content, keeping your QMS efficient, clear, and audit-ready.
Want to see for yourself how this works?