AOQuality (Quality Management System) for Life Sciences is the only end-to-end QMS that combines frictionless collaboration and automated regulatory validation with the intuitive Google Workspace user experience.
QMS.
Accelerated.
AOQuality for Life Science can be put in place in days with validated, prebuilt quality control templates and workflows, in compliance with HIPAA, FDA and ISO Guidelines.
Validated.
Always.
Achieve regulatory compliance from day one – FDA CFR Part 11, ISO 13485, and ISO 14971, and many more. AOQuality comes out of the box as a Qualified platform and a Validated library.
AI Insights.
Help your business identify and fix product issues faster thanks to AI-Driven dashboards and analysis of customer feedback. Speed up your QMS workflows by visualizing process bottlenecks.

Google Workspace Combined With AODocs Leads To Customer Success.
O8t needed a QMS that could address their document control needs and help them meet compliance requirements. Find out why they chose AODocs' solution for Life Sciences.


Ceva Animal Health is one of the world’s largest veterinary health companies, with over 5000 employees and offices in 42 countries. Find out how AODocs helped this pharmaceutical company digitize their registration process and reduce human error.
Read success story
A family business that was founded in 1853, Bonduelle helps people live well with great vegetables, grown and sold in 100 countries. Discover how Bonduelle chose AODocs to make sure information is always available to all employees, from the office to the field.
Read success story

As an emerging player in the life sciences space, Bio SB needed to implement a modern and more effective solution for document control.
Document Control
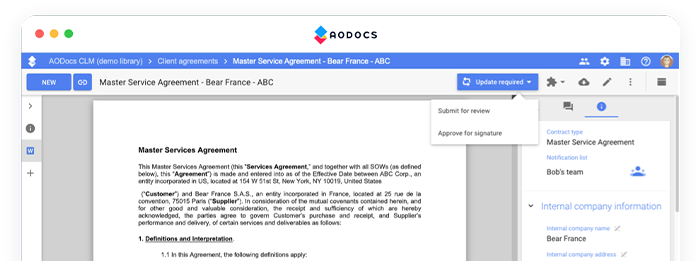
Have more control over the critical process documents that you rely on for your standard operating procedures, work instructions, and more. Do it all using the familiar collaboration tools found in Google Workspace, with apps like Google Docs, Google Sheets, and Google Slides.
Simplify Change Management
Seamlessly send documents through a regulatory compliant approval process and allow users to approve documents on their desktop or mobile apps.
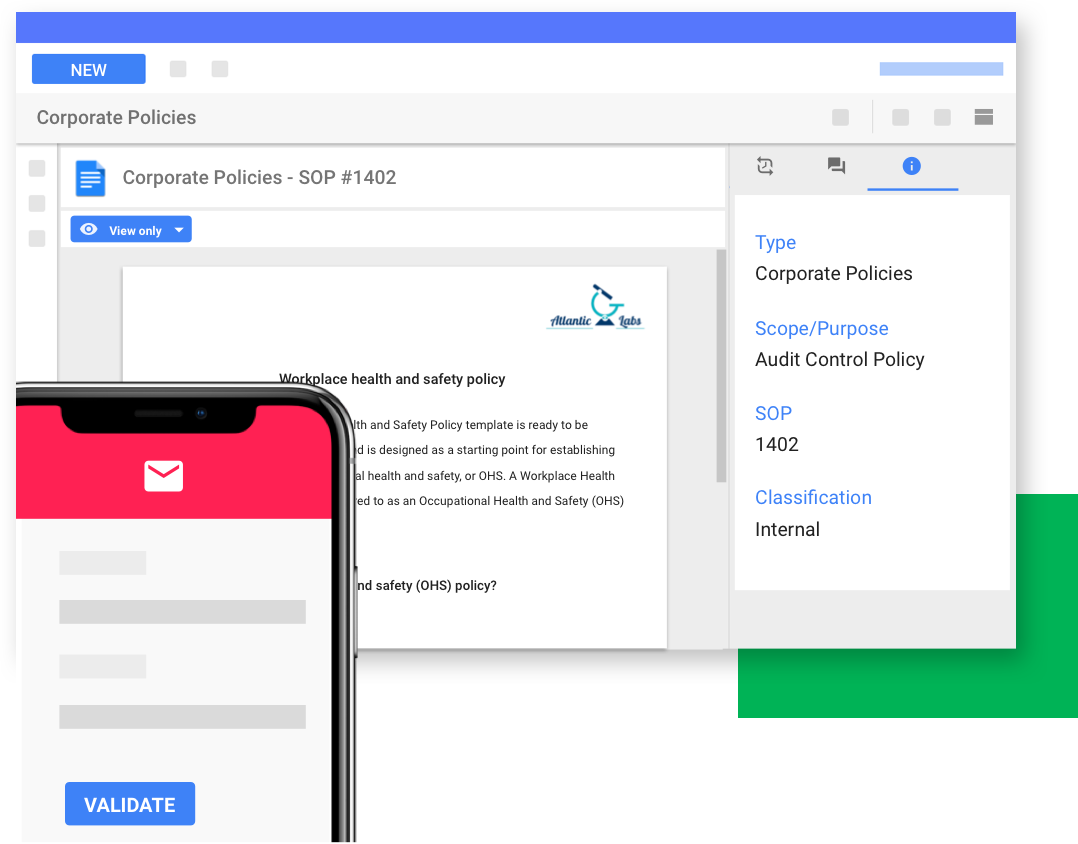
Say Goodbye to Manual Qualification and Validation
Regulatory Qualification and Validation with legacy systems takes a considerable amount of time, often taking days or even weeks to complete.
Remove those testing burdens with Validation as a Service.
Improve Your Audit Process
Store and track document metadata through the document lifecycle. Easily access all your request logs during compliance audits.

The Cloud-Based, Continuous Validation Solution That Exceeds Your Quality Management Needs
- Document Control
- Quality Records
- AI Driven Dashboards
- Human-Readable Audit Log
- Validation as a Service
- Dossier Cover Page
- PDF Renditions
- Corrective and Preventive Actions (CAPA)
- Audit Management
- Quality Events
- Testing & Acknowledgement
- Design Control Management
- Equipment Asset Management
- Supplier Management
- Business Intelligence and Reporting Dashboard
- Advanced Roles
- Integrations
- Customer Complaints AI Analysis
- Non-conformance Management
- Record Retention and Legal Holds
- Risk Management


.png)