Overcoming the year of the “confused copilot”
In 2024, many organizations launched Gen AI pilots, particularly those involving “Copilot” technologies for assistants and agents. However, many of these initiatives have yielded underwhelming results. The myriad “smart” assistants and “intelligent” agents were not mature enough to perform the required tasks efficiently and safely.
A common issue is that these AI systems often ingest information from various sources—emails, files, SharePoint sites—leading to a lack of control and potentially diluted productivity gains.
Beyond inefficiency, there are significant security and compliance risks to consider. Misconfigured access permissions can lead to sensitive files appearing in AI-powered search results. This vulnerability, which researchers titled “ConfusedPilot”, can have some practical and even dramatical consequences. Bloggers described a nightmare scenario where “employees casually stumble upon their CEO’s emails or private HR documents.”
So, organizations face enormous risks when over-relying on immature autonomous tools.
Making a Move: Obstacles to AI Production Deployment
Let’s assume you’d like to move these AI pilots into the more mature production phase, You want to reap productivity benefits before your competitors do. But which factors might be holding you back?
We at AODocs have identified two key obstacles:
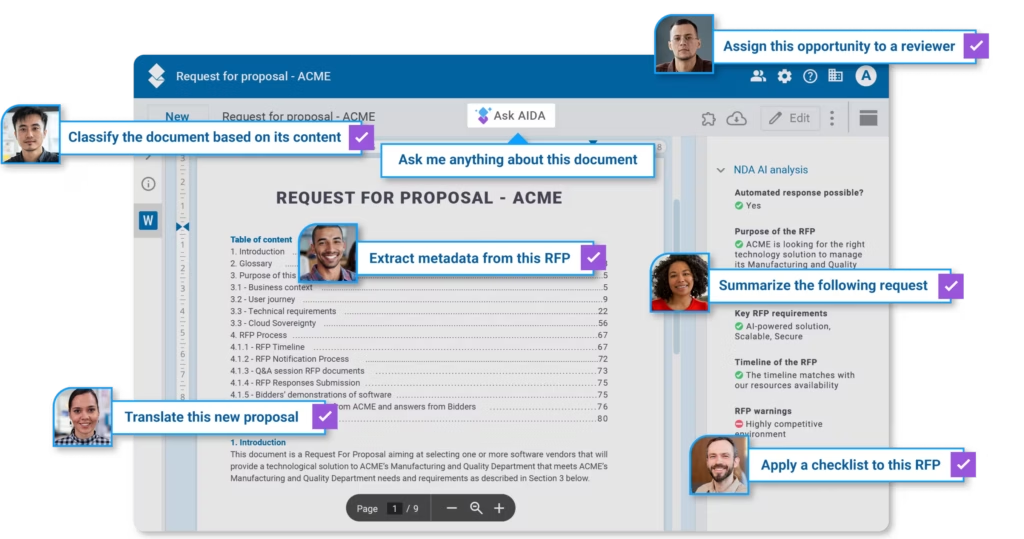
- AI Chatbot Chaos: Many companies have experimented with AI chatbots, often developed by internal teams. While managing knowledge sources for these chatbots may be straightforward during a pilot phase, production deployment presents lots of new challenges. Ensuring chatbots use only the most current, relevant, and secure documents is tricky when organizations don’t have automatic processes that channel the right information to the right stakeholders. How do you prevent your shiny new AI from leaking confidential information?
- Beyond Core AI Processing: Many AI pilots focus intensely on the AI processing itself—is the output accurate? But deploying these pilots at scale requires a solid process around the AI. This includes managing large document volumes, implementing human review workflows, ensuring traceability, and storing processed results.
Modern DMS systems can address these two categories of challenges.
2025: Moving Forward with Scalable Deployment
AI can be effective when deployed on controlled systems, such as a CRM, a contract repository, or a specialized document repository. Key principles for successful AI deployment include:
- Control the Data: Ensure AI tools access only approved and relevant documents.
- Process is Paramount: Implement robust document control processes before and after AI processing.
- Security First: Rigorously test security systems and access rights.
- Cross-functional Collaboration is Key: Foster collaboration between IT, developers, and business units.
Your next move: From AI Hype to Real-world Impact
We at AODocs provide solutions so that organizations can transition from AI pilots to production-ready deployments. AODocs helps control the documents used by AI, establish document processes, and ensure security and compliance. In 2025, AODocs aims to enable organizations to scale their AI projects and achieve real-world impact.
Discover how to take your AI initiatives from proof of concept to real-world impact with AODocs.