The best quality management program is one whose effectiveness is led by the people who practice it. As far as the adage “People, Process, and Technology” goes, the AODocs QMS solution with Validation as a Service for Life Sciences has brought all of these together, in a suite of features and functions that is just the right size for your quality program.
Software that offers quality management solutions for life sciences can be complex and difficult to implement for organizations that are trying to bring automation to their program for the first time. The manual processes these organizations have been relying on not only got the job done, but they were easy to understand, and the hands-on approach allowed them to see exactly what was going on.
But while it may work for you and your teams, effectively demonstrating these cumbersome legacy processes to regulators conducting an audit can be challenging. Also, what if your quality program expands to include more participants? Or what if additional regulations are tacked on? As program processes expand to incorporate more downstream and upstream requirements, the very nature of a manual process now becomes a burden, rather than an advantage. It’s like turning a cruise ship – it can be done, but it will take a lot of effort.
Get SaaSy: Cloud-Hosted is Critical
In today’s increasingly digital, increasingly remote business landscape, adopting a cloud-based quality management system (QMS) is a near non-negotiable in order to remain a contender in the Life Sciences industry, and to successfully scale your business. If you haven’t yet made the switch to a SaaS (Software as a Service) QMS, you may want to start here. If your organization has adopted cloud-based quality management software, but you need help ensuring quality and compliance for long-term success, look no further.
But as you grow your organization, your standard cloud-based QMS and former quality management processes may no longer suffice for complete quality assurance. With growth comes the need to expand WHO needs to participate in your quality program processes and contribute to the content. Managing onboarding and control of who has access to what, as well as the role of each participant in the content lifecycle, can become a barrier that prevents your coworkers, partners, and leadership from properly integrating your QMS program across the organization.
Why you need Validation as a Service
Navigating the regulatory landscape is a difficult feat for any life sciences company. Whether your organization is preparing for your next round of clinical tests, or inventing the next best medical device or software as a medical device, Validation as a Service is an integral part of all pharma and biotech business operations. Chances are, your business needs are changing at a rapid pace — and so are technologies. Relying on traditional QMS systems, the high cost of manual validation may prevent you from updating your company’s QMS more than once every few years. Validation as a Service provides your organization with automated product updates multiple times per year, all while ensuring your QMS remains validated and compliant at all times.
With Validation as a Service, you can rest assured that the processes occurring within your system are always compliant with FDA regulations including 21 CFR Part 11 and CAPA, alleviating validation burdens and allowing you to focus on delivering high-quality services and products.
The Google Cloud workspace infrastructure is constantly evolving, which makes traditional regulatory validation processes difficult to maintain. The AODocs platform architecture evolves with improvements to accommodate these structural changes. AODocs for Life Sciences is no different — our solution leverages a Validation as a Service framework to mitigate these risks and ensure that your AODocs libraries and applications are maintained in a continuously validated state. AODocs for Life Sciences with Validation as a Service is insurance that works to help organizations master their challenging regulatory landscape.
Create, Grow, Exceed: Customized offerings to help you scale
AODocs for Life Sciences’ latest feature enhancements include improved capabilities for document control, change management, training and acknowledgment, management of corrective and preventative actions (CAPAs), and audit management. The latest release also features capabilities for managing non-conformities, customer complaints, governance and retention, reporting and analytics, and more.
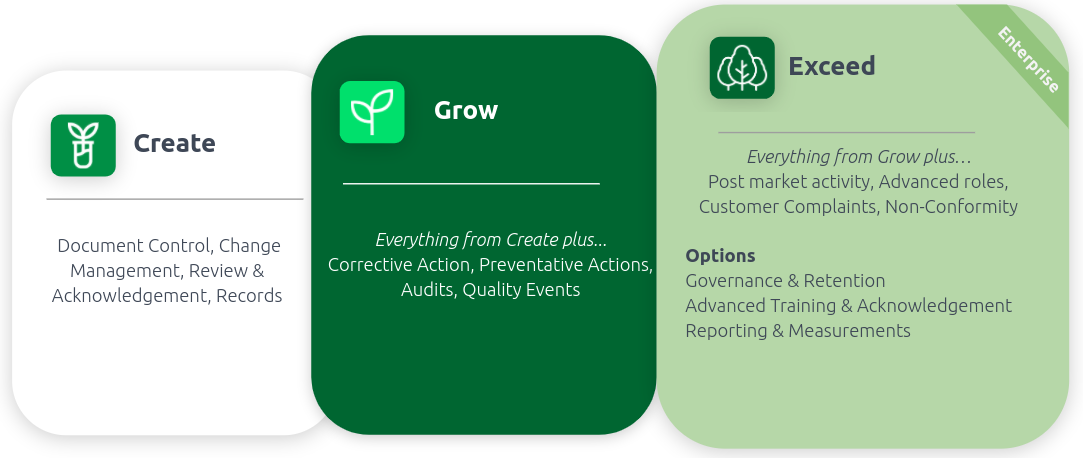
To discover the complete AODocs for Life Science package offerings, check out the infosheet below:
Speed to implementation, speed to change
The AODocs for life sciences QMS application allows you fast, easy access to all the critical information that you need, directly from the application, directly from Google Drive, or directly via the document you’re accessing. The intuitive user interface and seamless integration with your existing content management infrastructure means not only fast deployment, but high user adherence, even as you scale your teams.
AODocs QMS Solution for Life Sciences
AODocs for Life Sciences is a Quality Management platform designed to help medical device and pharmaceutical organizations comply with regulations while minimizing the burden of computer system validation, allowing them to focus on their core business and get their innovations to market faster.