Many organizations that have been around for a few years may already have “Quality” in place but it might be informal or incomplete. Others might not have even begun the process of developing a quality culture, but know that they need to “do something”.
The reality is that many companies need to develop a quality culture, and this is especially true with highly regulated industries like manufacturing.
While you could conceivably do all of this manually, you would be well advised to look for Quality Management System (QMS) software for manufacturing.
But before we dig into that, let’s first start with some QMS basics.
What is a Quality Management System (QMS)?
Simply put, a QMS is the connection and interaction of people, processes, and documentation within an organization. Think of a QMS as the glue that binds these things together. It is the collection of business policies, procedures, and functions designed to ensure customer expectations and requirements are met consistently every time. These outline how an organization will produce, document, control, and deliver all products and/or services.
While different types of manufacturers will have some variations in terms of regulatory oversight and the need to be compliant with those regulations as well as industry guidelines, there is going to be some kind of standard that will need to be met or exceeded. Moreover, you’re going to want to ensure that you have proof of this compliance, allowing your organization to be in an “audit-ready” state at all times.
Keep in mind that these regulations are not just going to focus on the products themselves. There will also be a need to meet or exceed OSHA stipulations as to workplace safety, the handling of specific types of materials, and a host of other aspects. The QMS software for Manufacturers should be capable of incorporating these elements into a complete QMS solution.
As I mentioned at the beginning of this post, you would be well advised to think through what you’re currently doing, and then take the time to understand how your business can develop a quality culture. This is more than a mission statement – a good quality culture should focus on preventing errors, ensuring compliance with regulations, while making improvements during the process.
This is an important step because you’ll then want to determine what aspects of that quality culture can then be best handled by a Manufacturing QMS platform. If your needs can’t be met by the QMS you may be considering, you’d be well-served to keep looking for one that can address your needs out of the box, or one that is designed to be customized to meet your needs.
While your specific need for a Manufacturing QMS may have some variations, in most cases, you’ll want to look for a QMS that can handle the following:
- Process Control and Risk Reduction
- Documentation
- Process Improvement
- Compliance with Regulatory Oversight and Industry Standards
- Supplier Management
Setting Up a Manufacturing QMS
Presuming that you have a solid grasp of your Quality needs and have selected what you feel to be the best QMS for Manufacturing, you now will want to set everything up. (You may want to work with a system integrator if you’re not comfortable doing this directly.)
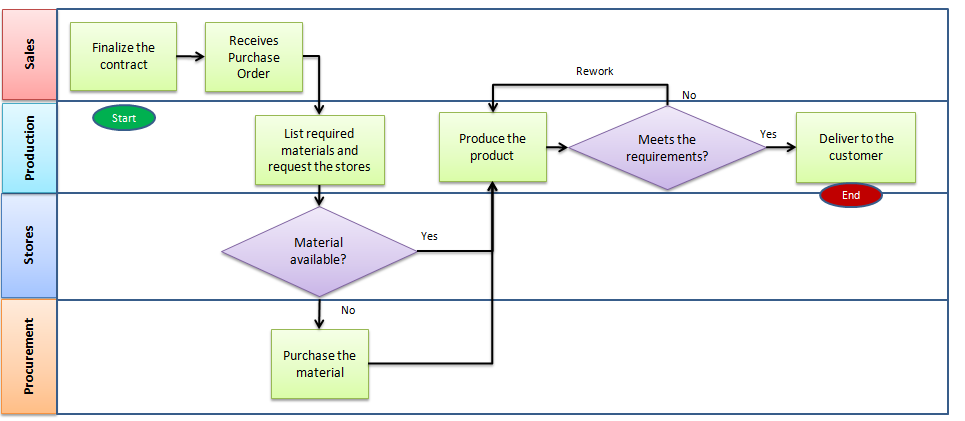
Map Out Your Manufacturing Processes
This is an essential step to setting up the QMS. If you’ve thought through your manufacturing and business processes as they stand now, or where you need them to be, now you’ll want to map all of the process steps required within the manufacturing facility.
Understanding the way the product moves through the pipeline allows for the identification of inefficiencies, risks, and where improvements can be put in place. This also shows where handoffs are, as these are notorious for causing issues to be resolved.
Mapping everything out with flowcharts makes it easy to visualize your processes and to identify those areas that could be improved or refined.
Once your processes are mapped then you can start understanding which ones of these steps are “critical to quality” or that have the most effect on the end product. An integral part of the QMS is process improvement, so ensuring the processes are efficient, and making improvements where needed, only makes the organization stronger.
Create Processes, Procedures, and Protocols
Now that you have the critical processes identified, you need to document them, whether that is a checklist, a work instruction, a set of standard operating procedures, or even something as simple as a manual.
To start the documentation process, putting a Document Management Solution in place is strongly recommended. This will ensure that everyone is working with the right version of a document, but it will also allow you to create governance and control who has access to any document or digital asset managed by the system.
Whatever you come up with – be it a set of policies, standards, protocols, or process manuals – will serve to ensure consistency and allow you to be compliant with regulations. Again, a Document Management System is a big benefit here, especially if you use different methods to convey the information, like videos, infographics, and flow charts.
If your text-based procedure manual gets an update, it’s a big plus if your system can flag all of the supporting materials that would also need a refresh. You’ll want these elements to be in sync at all times – you can imagine the confusion if a video says to do something one way, while a text-based document says to do something different.
Continuously Improve or Refine Your Manufacturing Processes and Operations
As manufacturing processes grow and change, including new technologies and tools, so must the processes be improved to ensure efficiencies are gained and risks are reevaluated with each change. Using tools like FMEA (Failure Modes and Effects Analysis) for risk analysis when running large batches brings many benefits.
Don’t forget about the maintenance of equipment. Tracking and scheduling preventative maintenance will ensure it is in good working order and prevent high costs due to a failure. Here, workflow automation could be used to alert when equipment is due for maintenance, or possible replacement.
One more thing to keep in mind is the employees. A Quality culture demands that they are trained to identify issues within the manufacturing process. Identifying these problems early and taking the steps to remedy them, will likely save a lot of costs down the line.
International Standards and QMS
I should mention that ISO 9001:2015 lays out the quality management system requirements. And is a good resource to follow even if you are not working towards a certification, as it addresses such things as development quality, supply chain management, and employee training to name a few. All of these things are needed for operations.
ISO 9001:2015 is the most common standard for any business using QMS, but additional standards apply to manufacturing. Some or all of these may apply to your operation:
- ISO 14000: Environmental management
- ISO 13485: Medical devices and equipment
- ISO/TS 29001: Gas and oil industry
- ISO/IEC 90003: Software engineering
- ISO 18091: Local government processes
- ISO 26262: Functional safety for automotive equipment
- IATF 16949 (replaces the ISO/TS 16949): Requirements for quality management systems for automotive OEMs and suppliers
TL;DR
A well-laid-out QMS in any organization helps cut costs. This is especially true in manufacturing. QMS is all about the control of processes and the reduction and mitigation of risks. When this is put in place properly, combined with a good quality culture and QMS software, it breeds an environment for continuous improvement along with ease of meeting regulatory standards, and ultimately, that builds a stronger organization.