Posted by Nagesh Nama ● 10/8/20 4:39 AM
Are Your Cloud Solutions FDA CSA Ready?
The Center for Devices and Radiological Health (CDRH), in cooperation with the Center for Biologics Evaluation and Research (CBER) and the Center for Drug Evaluation and Research (CDER), is preparing to release new guidance from the FDA in late 2020 for Computer Software Assurance (CSA) for Manufacturing, Operations and Quality Systems Software. The updated guidance from the FDA will provide guidelines for streamlining documentation with a focus on critical thinking, risk management, patient and product safety, data integrity, and quality assurance.
So, the big question many life sciences companies that produce FDA-regulated products are asking themselves is this:
How can we ensure we’re aligned with the FDA’s new best practices for software validation (which the FDA is now referring to as “assurance”)?
As the FDA prepares to release its new guidance later this year, life sciences companies should take a proactive approach for transitioning their current software validation methodology to one that more closely adheres to the tenets of a Computer Software Assurance approach that focuses on quality assurance, patient and product safety, and data integrity.
As we await the FDA’s release of its updated guidance, here’s some steps life sciences companies can take now:
- Embrace a “no paper” approach to validation soaked in automation. This is possible only if you adopt a sensible test automation framework which not only saves time but can increase your software assurance by a factor of 10X or more. With this, you can run your entire regression suite in minutes on a continuous basis.
- Build your test cases into the coding environment so that there is a direct traceability between the test scenarios and the test automation code (for example the Gherkins framework).
- Adopt a pipeline-based test deployment framework with built-in approvals. Such a framework can easily plug into your DevOps and provide all the audit trails to track every step in every run.
- Analyze the historical run data to see what the software validation scores are with every release.
Why Continuous Software Validation is Important for Life Sciences Companies
Digital transformation has kicked into high gear for most life sciences companies, and in this modern era, working with cloud vendors who can support a continuous software validation framework is no longer a “nice to have” – it’s a “we must have it.”
Cloud solutions must be validated for life sciences companies to use them, and every time a cloud vendor makes an update to their software (which can be frequently), it needs to be re-validated. Unfortunately, many life science firms still conduct validation testing manually, which is time consuming and prone to error.
Continuous validation approach alleviates the pain often involved with meeting validation requirements and helps provide the path to a smooth, secure, and compliant move to the cloud. There are numerous reasons why life sciences firms should commit to a continuous validation approach:
- Provides full coverage for patches and updates
- Provides full coverage for all layers (IaaS, PaaS, SaaS)
- Increases data integrity assurance
- Increases test coverage (built-in robust testing strategies)
- Provides built-in support for historical test data analysis
- Provides an adaptive framework designed to accommodate frequent requirement changes
- Reduces compliance risk (complies with 21 CFR Part 11 / Annex 11)
- Provides integrated risk and traceability
- It’s cost effective
How is it Done?
In a nutshell, here’s the continuous software validation process:
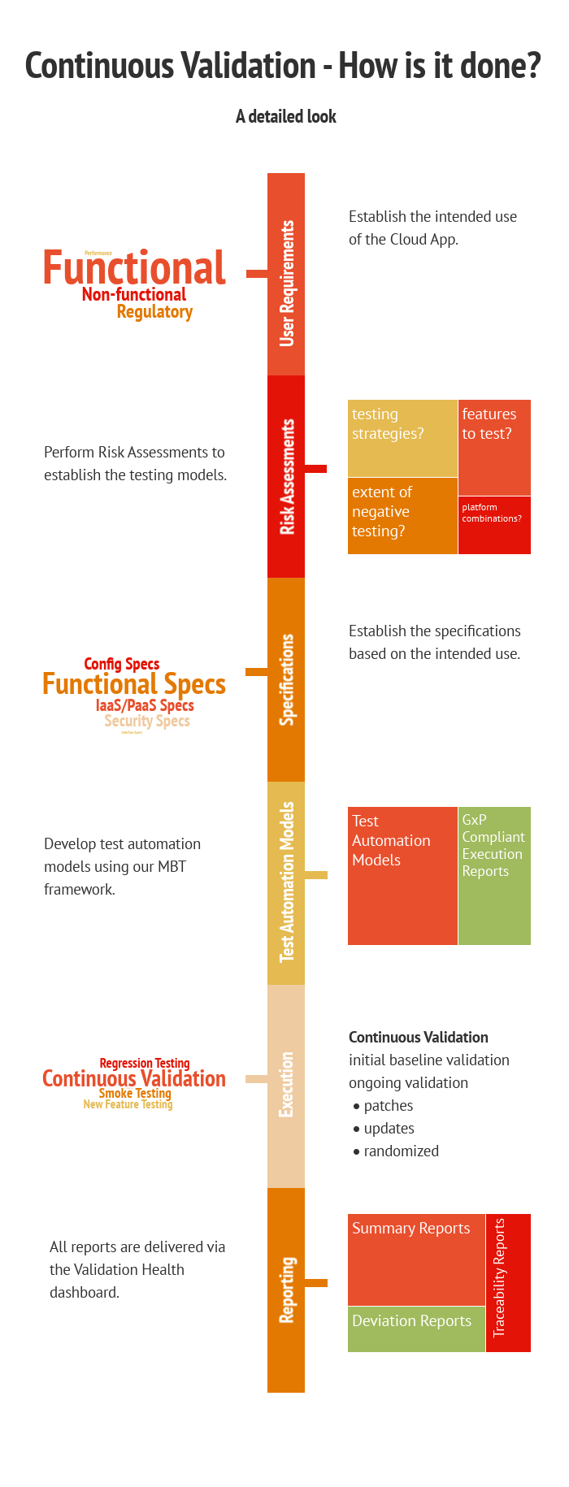 Source: xLM
Source: xLM
Digital transformation is proliferating in the life sciences sector as new technologies and validation approaches are delivering heightened levels of quality and assurance. Now is the time to modernize your firm’s software validation and assurance approach!
To learn more about continuous software validation, check out the xLM and AODocs How to Navigate 21 CFR Part 11 Compliance in the Cloud webinar recording, or download our infosheet, AODocs for Life Sciences Continuous Validation.
Tags: Compliance



